Abstract
Background
The use of novel Multiple Myeloma (MM) drug agents has been associated with improved therapeutic outcomes and survival; however, MM continues to pose a significant economic burden on both patients and healthcare systems. Evaluating the economic implications and outcomes of available treatments is important to improve efficiency of care.
Patients with MM may experience productivity loss, including lost days from work or inability to work due to MM symptoms or to undergoing treatment. Although direct costs of illness have been well described in the literature, indirect costs associated with MM are understudied. Here, we examine the extent of workplace productivity loss among U.S. adult patients with MM and associated costs. These outcomes were compared among patients who received injectable versus oral MM therapy.
Methods
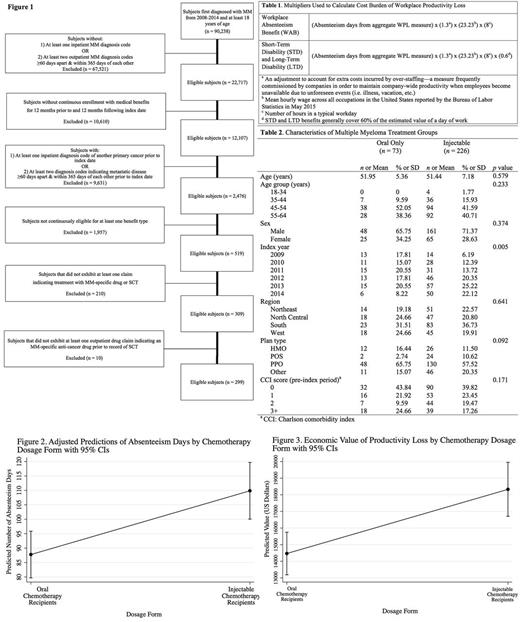
Analyses were conducted using the Truven Health Analytics MarketScan Commercial Claims and Encounters with Medicare Supplemental Coordination of Benefits and Health and Productivity Management Databases. Subjects at least 18 years of age diagnosed with MM between January 1, 2008 and December 31, 2014 were included in our study and followed until December 31, 2015 (Figure 1). Subjects were analyzed one year prior to, and one year following first MM diagnosis. Patients receiving only oral therapy were compared with those who received injectable therapy.
Productivity loss was evaluated using several key variables found within workplace absenteeism (WAB), short-term disability (STD), and long-term disability (LTD) claim files. Workplace productivity loss (WPL), an aggregate of absenteeism days, was calculated using claims for WAB, STD, and LTD benefits. Cost of productivity loss was calculated for each patient by multiplying the number of absenteeism days (as indicated in the aggregate WPL measure) by the average daily wage for all occupations, as reported by the U.S. Bureau of Labor Statistics (Table 1).
Absenteeism days and costs were compared among study groups using multi-variable zero-inflated Poisson regression. The following variables were used as covariates in the model: age, index year, gender, Charlson comorbidity index, and plan type. An interrupted time-series analysis was also conducted to evaluate changes in productivity losses in the pre- vs. post-diagnosis periods, and to compare these among the treatment groups.
Results
The final study cohort included 299 patients with newly diagnosed MM, of whom 73 received oral therapy and 226 received injectable therapy. Baseline characteristics of the study groups are depicted in Table 2. In the zero-inflated Poisson regression, treatment type was a significant predictor of productivity loss (Figure 2). Patients who received injectable therapy missed an average of 110 work days in the one year post-diagnosis, compared with 88 for patients receiving only oral therapy (difference of 22 days, 95% CI: 19 - 25, p<0.001). Treatment type was also a significant predictor of cost of lost productivity in this model (Figure 3). Patients who received injectable therapy experienced productivity loss valued at $18,329, compared with patients who only received oral drug therapy ($14,467). The difference in valuated productivity losses between study cohort groups was also statistically significant ($3,862, 95% CI: $3,518 - $4,205, p<0.001).
An interrupted time-series analysis showed a significant increase in missed work days per month following the initial diagnosis of MM for both treatment groups. Patients receiving injectable therapy experienced an immediate increase of 6.9 lost productivity days per month (95% CI: 5.1, 8.7, p<0.001), versus patients receiving oral therapy only (4.4 days per month; 95% CI: 2.7, 6.2, p<0.001). The difference in these values was not statistically significant, although a strong trend was observed (2.5 day per month; 95% CI: -0.1, 5.0, p=0.057).
Conclusions
In conclusion, patients newly diagnosed with MM face significant losses in productivity. Injectable MM therapy users experience significantly more utilization of disability benefits and incur higher productivity costs, compared to oral MM therapy users. Further studies elucidating the nature of the differences between parenteral and non-parenteral chemotherapy users are needed.
Merola: Millennium Pharmaceuticals, Inc.: Consultancy. Yong: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Noga: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Shermock: Millenium Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal